
The latest news from ItaCRIN and its partners
News
Independent Research Call 2025 – Antimicrobial Resistance and Precision Medicine
Thursday, October 23, 2025,
The second Independent Research Call 2025 allocates €20 million to support independent scientific research aimed at generating new evidence on medicines with potential impact on the Italian National Health Service.
The call is open to all Italian researchers affiliated with public or private institutions who intend to conduct non-commercial studies.
Research proposals must fall within one of the investigation lines defined for the following thematic areas:
- Antimicrobial Resistance
- Precision Medicine
Applications can be submitted from 12:00 PM on Monday, 15 September 2025 until 12:00 PM on Tuesday, 18 November 2025.
For eligibility criteria and application procedures, the full Call text and its annexes—including the PI User Manual and FAQs—are available here.
Don't miss the last issue of our Newsletter!
Wednesday, March 26, 2025,
View here the latest news and breaking news from the Italian Network on the Spring Issue of our Newsletter.
Season's greetings from ItaCRIN!
Thursday, December 19, 2024,

ECRIN launches the new tool "RED"
Wednesday, October 16, 2024,

We are thrilled to announce the official launch of RED (Regulatory and Ethical Database)—a tool designed by ECRIN to provide clinical researchers with comprehensive information on clinical study regulatory information and ethical submission requirements across Europe.
You can access RED here: https://red.ecrin.org
2 new Clinical Trial Centers joined ItaCRIN Network!
Wednesday, September 4, 2024,
Our Network is growing fast!
It is our great pleasure to announce that the Clinical Trial Center of Azienda Ospedaliero Universitaria Careggi (Florence) and the Clinical Trial Center of Azienda Ospedaliero Universitaria SS Antonio e Biagio e Cesare Arrigo (Alessandria) became part of ItaCRIN.
Welcome on board 😀
canSERV Second OPEN CALL for "Transnational Service Provision"
Tuesday, March 5, 2024,

The mission of the canSERVs project is to make cutting-edge and customised research services available to the cancer research community EU wide, enable innovative R&D projects and foster precision medicine for patients benefit across Europe. By connecting, coordinating, and aligning existing oncology and complimentary research infrastructures (RIs) and providing services in a synergistic way transnationally, canSERV will capitalise on the critical mass of experts and cutting-edge services offered by canSERVs RIs and their extended network.
📣 The second call is OPEN! APPLY NOW for the @canSERV Second OPEN CALL for "Transnational Service Provision"!
Do you want to accelerate your research project and profit from free access to 400+ #CancerResearch Services in 10 research fields provided by 13 #ResearchInfrastructures?
Apply via: https://lnkd.in/eX3q6KcY
the 4th issue of the ItaCRIN Newsletter is now online!
Monday, December 11, 2023,
Catch up on the latest news in the December ItaCRIN Newsletter: https://www.itacrin.it/newsletters
Open Position - Project Manager
Wednesday, September 13, 2023, Rome
A position will soon be open at the Scientific Directorate of the Regina Elena Institute, by means of a public call for applications for a one-year contract, eventually renewable, as Project Manager to support the management of European research projects of the Cancer Mission (https://research-and-innovation.ec.europa.eu/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe/eu-missions-horizon-europe/eu-mission-cancer_en) and of the Cancer Beating Plan (https://www.ipaac.eu/news-detail/en/53-europe-s-beating-cancer-plan-a-new-eu-approach-to-prevention-treatment-and-care ). The successful candidate will be in charge of managing interactions with European networks in which the institute is involved or will be involved in the future, driving the activities foreseen by the projects, controlling their preparation and implementation, monitoring results, coordinating technical reporting, maintaining relations with network members and ensuring the continuity of the reporting process. In order to carry out its functions it will have to interface, from time to time, with the experts of the Regina Elena Institute necessary for the various project requests, coordinating ad hoc working groups interfacing with the Scientific Direction and the Grant Office. Among the activities foreseen, but not limited to these, the chosen candidate will have to actively participate, as a representative of the Regina Elena Institute, in the preparatory meetings of the project proposals to be presented, in the kick-off meetings and in the periodic meetings of the different projects. The envisaged professional figure should have a scientific background possibly coupled with previous experience in the field of international relations. High familiarity with cloud portals for document sharing such as Onedrive/Sharepoint is required. Excellent knowledge of the English language is required. A knowledge of project management techniques in the field of research, particularly in oncology, will be an advantage.
📌 For further information, interested candidates may contact Giulia Piaggio at 06-52662458 or by email giulia.piaggio@ifo.it or giulia.piaggio@gmail.com
📬 The 3rd ItaCRIN Newsletter issue is now available!
Monday, July 10, 2023,
In this issue:
🇮🇹 News from the Italian Network
📰 ECRIN breaking news
🗓️ Past events
📔 Upcoming events for your agenda
To read the full newsletter, please click here 👉 https://mailchi.mp/3f42e3838ee9/itacrin-newsletter-3rd-issue-9581410
⏱️ This newsletter is a ten-minute read.
Successful Course on Sensitive Biomedical Data
Monday, June 5, 2023, Rome

On May 25-26, the training "FAIRifying sensitive data: technical, legal and practical considerations in the biomedical field" was organized by the Italian National Institute of Health (ISS) with funding from EOSC-Life. Participants learned about the principles of biological and medical sensitive data and medical applications, access to data resources and data FAIRification (especially for health research and health care data).
The training was very participated!
Greece is a new Member Country of ECRIN
Monday, May 22, 2023,

We are very proud to announce that Greece has officially joined ECRIN-ERIC as a Member country. The hub of the Greek network, GreCRIN, will be hosted at the Centre for Research and Technology, Hellas (CERTH) in Thessaloniki.
ECRIN has worked with Greek scientific partners on various projects, and ECRIN’s Operations Director, Christine Kubiak, is extremely delighted to take this collaboration to the next level. Working together with the Greek scientific community, and building a structuring Greek network for multinational clinical research is in line with ECRIN’s mission and vision.
With Greece joining ECRIN as a Member country, ECRIN now has a total of 13 Member and Observer states. ECRIN’s network now counts: Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, Norway, Poland, Portugal, Spain, Slovakia and Switzerland. The total reach of the ECRIN network has grown to over 361 million European citizens, with an average of 6.5 countries per trial.
Get to know ECRIN National Partners
Wednesday, February 15, 2023,
In 2023 ECRIN will dedicate one month to each of our 12 national partners.
They started in January with CZECRIN to continue with French Partner: F-CRIN.
See the post about CZECRIN & F-CRIN on social medias and more information on the latest news on ECRIN website
Innovative Health Initiative launches first calls for proposals
Friday, July 1, 2022,
IHI’s first calls are a fantastic opportunity for health researchers and stakeholders to work together on pressing issues that can only be addressed by a cross-sector, multi-stakeholder partnership.
The topics cover diseases such as cancer, cardiovascular disease and neurodegenerative diseases, as well as cross-cutting issues like health data and early stage studies of medical devices.
For more information, please visit 👉 https://www.ihi.europa.eu/news-events/newsroom/innovative-health-initiative-launches-first-calls-proposals
Clinical Trials Information System (CTIS) go live
Tuesday, February 1, 2022,
On Monday 31st January 2022, the Clinical Trials Information System (CTIS) has gone live. CTIS is the backbone of the Clinical Trials Regulation that will harmonise the assessment and supervision of clinical trials in the EU and the EEA.
The Clinical Trials Regulation and CTIS represent one of the most ambitious regulatory projects of the European medicines regulatory network. The development of CTIS involved the joint effort and dedication of Member State national competent authorities and ethics committees, clinical trial sponsors representing commercial enterprises (including SMEs) and academia, representatives of patients and healthcare professionals and members of the general public, EMA staff and contractors, the European Commission and HMA. The Clinical Trials Regulation and CTIS will strengthen clinical trials in the EU, ensuring better outcomes for patients and supporting the attractiveness of the European Union as a location for clinical research.
Sponsor users can read the User Access Management Quick guide for information on getting started with CTIS. Users are encouraged to review the CTIS online modular training programme for information on how to use CTIS. Sponsors can also consult the CTIS Sponsor Handbook. Sponsors are reminded that they can make use of a transition period for submitting clinical trials to CTIS.
Patients, healthcare professionals and the general public can also access the public Clinical Trials website at euclinicaltrials.eu.
New ECRIN logo
Monday, November 29, 2021,

We are pleased to share with you that ECRIN launched its new corporate identity – logo and supporting colours.
You can find all the necessary information here 👉 https://ecrin.org/ecrin-brand-guidelines.
Launch of European Covid-19 trials coordination module website
Monday, June 28, 2021,
The coordination module of theEU-funded projects RECOVER1and EU-RESPONSE2has launched its website https://covid19trials.eu/en. It centralises information for academics and industry on access to the three large Pan-European adaptive platformtrials currentlyunderway: DisCoVeRy, EU-SolidAct & REMAP-CAP. A coordinated Pan-European approach is essential for tackling the COVID-19 epidemic, and finding the most effective therapeutic solutions. EU-RESPONSE and RECOVER, share a joint coordination module that allows them to evaluate potential therapeutics forCOVID-19,while avoiding duplications and maximizing the use of resources. The coordination module, composed of the Trial Coordination Board (TCB), the Joint AccessAdvisory Mechanism (JAAM) and the Adaptive Platform Trial Toolbox,ensures optimal coordination of trials in the EU and abroad and provides a single entry pointfor new study arms in the European COVID-19 adaptive platform trials.This module is coordinated by ECRIN and the Norwegian Institute of Public Health. The dedicated website describes the different bodies that aid in the coordination of the COVID-19 clinical trialsand therolesthey havebeen conferred. It clearly outlines the specificities of the different trials and how researchers and industry can applythrough the JAAM. The JAAM is the entryway for the European COVID-19 adaptive platform trialsalready implanted inover16European countriesand beyond. It is the single body, common to the DisCoVeRy, EU-SolidActand REMAP-CAPtrials, that assesses requests from investigators or industry looking to test their compound in these trials.The website also contains resources that will helpresearchers looking to develop platform trials navigate the complexities of this novel trial methodology.
Platform trials
Platform trials, allow a multitude of different treatment options (arms) to be compared with a single control (arm). They enable ineffective arms to be stopped after an interim analysis and new treatment arms to be added and may run in a perpetual manner. To date, they have been used principally in the field of oncology and more recentlyforinfectious diseases.
Contact information
For more information visit the covid19trials website, or contact the coordination module covid19trialseu@ecrin.org
1. EU-RESPONSE is supported by the European Union’s Horizon2020 Research and Innovation programme under Grant Agreement number 101015736.
2.RECOVER is supported by the European Union’s Horizon 2020 Research and Innovation programme under Grant Agreement number 101003589.
EU SolidAct - a pan-European platform for pandemic research and preparedness
Friday, May 28, 2021,
There is an urgent need for rapid and coordinated investigation of new candidate drugs during pandemics. EU-SolidAct is an Adaptive Platform Trial developed to evaluate drug interventions in hospitalized patients with COVID-19 and to facilitate a joint European response to future pandemics. The first drug to be tested is baricitinib, a tablet prescribed for treatment of rheumatoid arthritis. This drug has also shown promising results for patients with severe COVID-19. EU-SolidAct will recruit patients from around fifteen European countries, and will open the first sites in Norway and France the coming week. The trial is sponsored by Oslo University Hospital, Norway. EU SolidAct is part of EU-RESPONSE, a five-year project coordinated by Inserm, France. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101015736.
Contact: Associate professor Marius Trøseid, Oslo University Hospital, +4792440240, marius.troseid@medisin.uio.no
ItaCRIN 2nd Annual Meeting
Monday, April 26, 2021,
Our second Annual Meeting took place virtually on April 22nd. Several presentations were made on ongoing clinical studies and projects submitted in the past calls, the impact of Covid-19 on clinical trial research, the communication plan and the next steps in 2021 focused to further improve the impact of ItaCRIN in both the National and International landscape. A very participated discussion followed each topic.
The ItaCRIN Team would like to thank you all participants.
To find out more about this meeting, don't miss our next news!
Don’t miss the last COVID-19 Clinical Trial Report!
Thursday, April 22, 2021,
During the last year, from the beginning of the Covid-19 pandemic, the COVID-19 Clinical Trial team of the National Institute of Health (ISS) periodically published Clinical Trial Reports related to Coronavirus emergency.
With this update, the work of the WG is concluded. Through 9 infographics, they provided an overview of the global and national-level COVID-19 interventional pharmacological clinical trials, highlighting key data and trends. There was also a section dedicated to the vaccines against COVID-19 including valuable insights into those ones in clinical phase.
To see the last and all the past published issues, please click here 👉 https://www.epicentro.iss.it/coronavirus/sars-cov-2-analisi-studi-interventistici
ECRIN launches 2021-2023 Strategic Plan
Thursday, April 15, 2021,
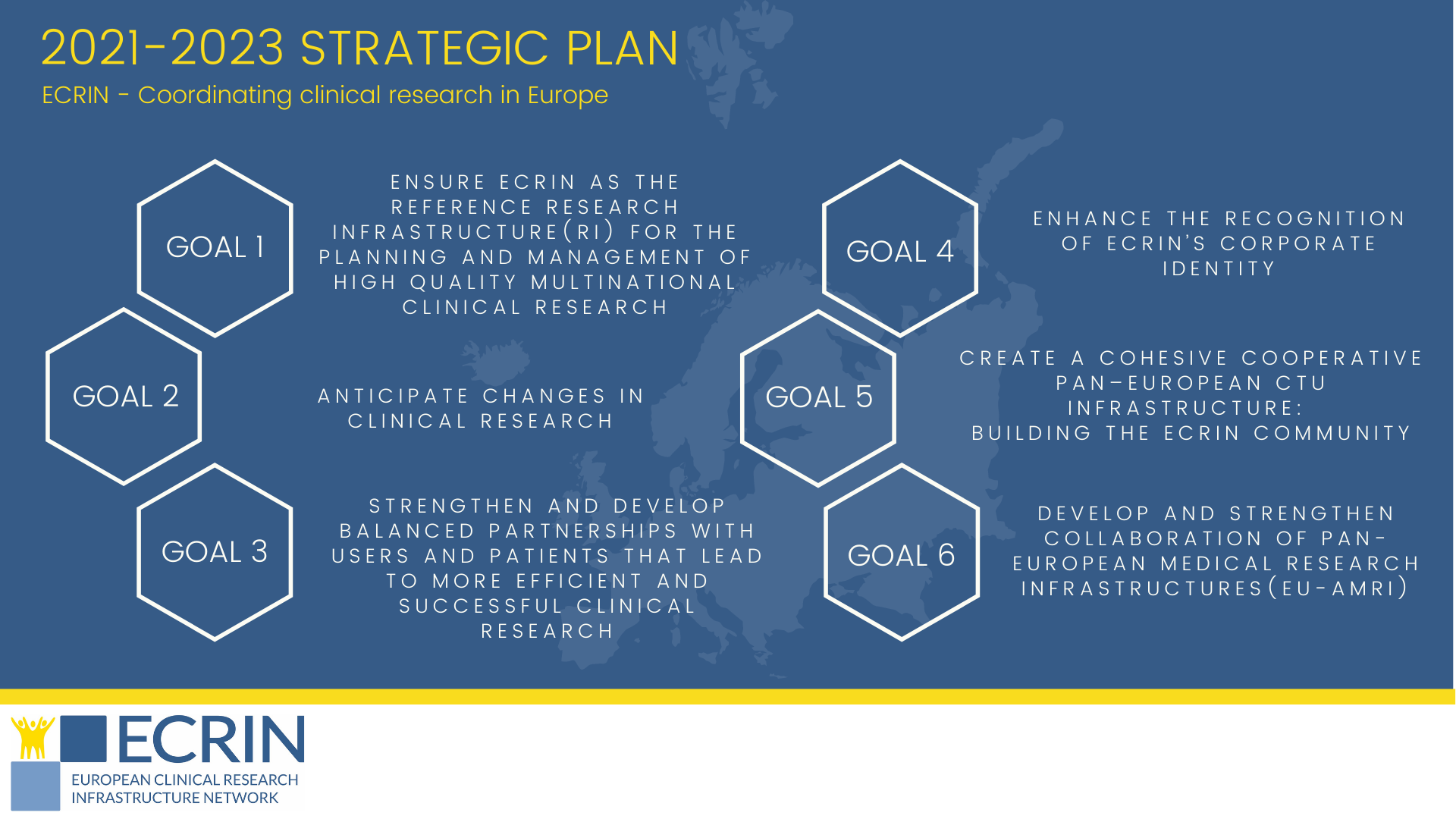
This strategy sets out ECRIN’s priorities over the next three years. It defines the six areas of focus for ECRIN and carves out a path to advance our mission and vision. For each of the goals set out in this plan, distinct actions have been identified that will be carried out over the next three years.
Through the strategic plan, ECRIN looks to answer, even anticipate the users’ needs, be leading-edge as well as recognised as the reference in the setup and management of multinational clinical trials in Europe.
View the ECRIN 2021-2023 Strategic plan.
New Job Positions
Friday, March 26, 2021, France
Looking for a job in Clinical Research?
Please explore the list of professional vacancies currently available in ECRIN.
Visit the dedicated page to find the available job vacancies 👉 https://ecrin.org/who-we-are/jobs-list
Call for renewing the Independent Certification Board membership
Wednesday, March 17, 2021,
Within the framework of ECRIN Data Centre Certification 2021 workplan, it has been launched a call for renewing the Independent Certification Board membership.
The Independent Certification Board is currently including 6 expert members.
Taking into account that the ISO 9001:2015 certified set of ECRIN services is continuously growing with more interest expressed by EU data centres for being certified and a confirmed recognition of the programme value for academic data centres expressed by the EMA, ECRIN wishes to trigger a call for ICB membership application and considers the extension of the Board to 2 more members. This will also contribute to ensure a quorum of voting members at ICB meetings, hence decreasing delays in the certification evaluation.
The deadline for receiving applications is April 23th, 2021. If interested, please follow the link to the application:
👉 https://survey.ecrin.org/surveys/?s=494Y4J8A3F
Candidates for International Rare diseases research consortium
Thursday, February 18, 2021,
The IRDiRC consortium (International Rare diseases research consortium 👉 https://irdirc.org/) is looking for candidates for two separate Task Forces. The Task Forces are:
a. Integrating New Technologies for Diagnosis of Rare Disease-s Patients
b. Shared Molecular Etiologies Underlying Multiple Rare Diseases.
The application deadline is March 1.
For more information they are currently news 2&3 on their news feed
The 3rd EJP RD – JTC 2021 is OPEN
Thursday, January 28, 2021,
The 3rd EJP RD Joint Transnational Call for Rare Diseases Research Project (JTC 2021) focuses on “Social sciences and Humanities Research to improve health care implementation and everyday life of people living with a rare disease”.
There will be a two-stage submission procedure for joint applications: pre-proposals and full proposals.
An independent international Scientific Evaluation Committee will carry out a scientific evaluation according to specific evaluation criteria.
Preliminary timetable
16th February 2021 Pre-proposal submission deadline
End of April 2021 Invitation to full proposal
15th June 2021 Full proposal submission deadline
30th July 2021 Deadline for rebuttals
November 2021 Notification of funding decision
For more information, please visit the website 👉 https://www.ejprarediseases.org/index.php/ejprd-jtc2021/
TBMED Open Call: Support for CPoC or clinical investigations
Friday, January 22, 2021,
The TBMED Open Call has just launched an Open Call with the objective of providing support to developers which aim to perform a Clinical Proof of Concept (CPoC) or clinical investigation with an innovative high risk medical device.
Deadline for submission of applications: February 22, 2021 23:59 CET
Here is the link to the website:
https://tbmed.eu/news/tbmed-open-call-support-for-cpoc-or-clinical-investigations
Don’t miss the last COVID-19 Clinical Trial Report of the year!
Tuesday, December 22, 2020,
In the 8th issue you may find a section dedicated to the Covid-19 phase III vaccines.
Which are the four authorised vaccines around the world?
Click to read the full report 📊
Season's Greetings from ItaCRIN
Monday, December 21, 2020,

A new ECRIN Newsletter is now online!
Wednesday, December 16, 2020,
Don't miss the latest news at ECRIN with the last december newsletter: https://ecrin.org/our-newsletters
ECRIN awarded ISO 9001:2015 certification
Friday, December 11, 2020,
The 7th issue of COVID-19 Clinical Trial Report is now online!
Thursday, November 26, 2020,
The COVID-19 Clinical Trial report series is going on to update you with current data and insights on clinical trials authorized throughout the COVID-19 pandemic.
In this issue, the COVID-19 Clinical Trial team of the National Institute of Health (ISS) dedicated a section to the Italian studies with convalescent plasma and a special focus on the TSUNAMI Study, whose sponsors are ISS and the Italian Competent Authority, AIFA.
Click here to view the report 👉https://www.epicentro.iss.it/coronavirus/sars-cov-2-analisi-studi-interventistici
PRE-ANNOUNCEMENT JTC 2021
Friday, October 23, 2020,
ERA PerMed is an ERA-Net Cofund, supported by 32 partners of 23 countries and co-funded by the European Commission. To align national research strategies, promote excellence, reinforce the competitiveness of European players in Personalised Medicine (PM), and enhance the European collaboration with non-EU countries, 28 funding organisations have agreed to launch the fourth Joint Transnational Call for collaborative innovative research projects in PM.
The funding organisations participating in this call particularly wish to promote innovative interdisciplinary collaboration and to encourage translational research proposals.
The call is planned to be launched on December 14th 2020 with a submission deadline for pre-proposals on March 4th 2021. It is expected that consortia invited for the full-proposal stage, will need to submit their proposal on June 16th, 2021.
Download the full text of the ERA PerMed JTC2021 pre-announcement here (updated 16 Oct 2020) 👉 PRELIMINARY ANNOUNCEMENT JTC 2021
ECRIN signed the Manifesto for EU COVID-19 Research
Friday, October 16, 2020,
The Commission has launched a manifesto to maximise the accessibility of research results in the fight against COVID-19.
The Manifesto provides guiding principles for beneficiaries of EU research grants for coronavirus prevention, testing, treatment and vaccination to ensure that their research results will be accessible for all and guarantee a return on public investment.
Don't miss the 6th issue of COVID-19 Clinical Trial Report!
Thursday, October 15, 2020,
The 5th issue of COVID-19 Clinical Trial Report is now available!
Thursday, September 17, 2020,
Announcing EOSC-Life's Digital Life Sciences Open Call
Tuesday, September 15, 2020,
EOSC-Life has launched its first Digital Life Sciences Open Call: A European Open Science Cloud (EOSC-Life) call for projects sharing data, tools and workflows in the cloud.
This call offers financial support for projects enabling life science researchers to connect their research to the cloud, alongside with training, advice and assistance from data experts, tool developers and cloud specialists.
Deadline for proposal submission is 22 December 2020.
Find out more about eligibility, application and review processes and budgeting by visiting www.eosc-life.eu/opencall.
ECRIN Annual Report 2019 is now available!
Friday, August 7, 2020,
We are excited to announce that the ECRIN Annual Report 2019 is now available on ECRIN website.
See the report on how we support multinational Clinical Trials and Infrastructure development projects to enhance medical practice and to improve public health to this link: https://ecrin.org/sites/default/files/annual%20reports/Annual-Report-A4-ECRIN-2019.pdf.
A Swedish SMO interested in conducting a multinational clinical trial to test a medical device for depression is seeking collaboration with ECRIN!
Wednesday, August 5, 2020,
Located in Sweden, Flow Neuroscience provides a CE-certified complete at-home depression treatment combining a transcranial direct current stimulation (tDCS) headset with a behavioural therapy application.
Flow is currently seeking research partners in the EU to conduct a multinational clinical trial program on the efficacy of our product in the treatment of anxiety. Flow, together with the research partners, will apply for the Horizon2020 European Commission grant (EIC Accelerator Pilot - up to €2.5M funding) that is dedicated to fund early-stage innovations. To orchestrate the multinational clinical trial program, we are also seeking collaboration with ECRIN.
In the partnership, Flow will be solely responsible for the preparation of the grant application. Clinical studies under the partnership will be fully funded by the grant (expected launch: Q3/Q4 2021).
If interested in discussing this potential research collaboration, please contact us!
📬 elena.toschi@iss.it; maria.buoncervello@iss.it
July 2020 ECRIN newsletter is now online!
Monday, August 3, 2020,
Don't forget the ECRIN's July 2020 edition of its newsletter. This issue includes updates on infrastructure development projects, National partners, COVID-19 Taskforce, and more.
Don’t miss the 4th issue of COVID-19 Clinical Trial Report!
Thursday, July 30, 2020,
In the new issue, the Clinical Trial working group of ISS is keeping a close eye on evolving Covid-19 vaccines.
Read more about the 4th issue here
See you with the next report after summer break!
The 3rd issue of COVID-19 Clinical Trial Report is now online!
Thursday, July 9, 2020,
We continue the COVID-19 Clinical Trial Report series – bringing you current data on clinical studies approved in Italy and around the world.
Click to view the new report, past reports and methodological note.
Join the 2nd issue of COVID-19 Clinical Trial Report!
Friday, June 19, 2020,
Don't miss out on the 2nd Edition of issue of COVID-19 Clinical Trial Report.
To view and read the report, please visit the link https://www.epicentro.iss.it/coronavirus/sars-cov-2-analisi-studi-interventistici
COVID-19 Clinical Trial Report
Friday, June 12, 2020,
Five practical steps to accelerate COVID-19 research
Monday, June 8, 2020,
ECRIN, together with EATRIS and BBMRI, published top 5 recommendations to accelerate COVID19 research.
Research Data Alliance COVID-19 recommendations and guidelines
Wednesday, June 3, 2020,
The Research Data Alliance recently published the pre-final version of their guidelines and recommendations on data sharing in the context of COVID-19. An open consultation period has begun which will close on 8 June.
Please see attached a cover statement providing some context and the links to both the guidelines and the website through which to provide feedback.
They encourage to share this cover statement widely among communities to ensure that as many stakeholders as possible have the opportunity to shape this very useful resource.
Feedback can be provided by following this link: https://www.rd-alliance.org/group/rda-covid19-rda-covid19-omics-rda-covid19-epidemiology-rda-covid19-clinical-rda-covid19-0
Clinical Research Metadata Repository launched
Thursday, May 7, 2020,
International Clinical Trials Day 2020 cancelled
Monday, May 4, 2020,
Location: Berlin, Germany
A new CTU joined ItaCRIN Network!
Wednesday, April 22, 2020,
COVID-19 Data Portal
Tuesday, April 21, 2020,
BBMRI, ECRIN AND EATRIS against COVID-19 pandemic
Friday, April 10, 2020,
The three Research Infrastructures are working together under the umbrella of the Alliance of Medical Research Infrastructures (AMRI).
The full catalogue of AMRI resources is available to the COVID-19 research community.
For more information please follow the guidance provided here or contact EATRIS Business Development Manager directly: christieken@eatris.eu; +31(0) 6155 66487 on how to access to the services.
TRANSVAC2 offers a support to the development of vaccines against COVID19
Wednesday, April 8, 2020,
TRANSVAC supports innovation for both prophylactic and therapeutic vaccine development. High quality technical services across four different service platforms are offered: Technology (for process development and GMP production), Immunocorrelates & Systems Biology, Animal models, and support for Clinical Trials. Academic and non-academic research groups, including SMEs, can apply to benefit from the expertise, reagents, and facilities offered by TRANSVAC2 to accelerate the development of their vaccines.
ECRIN contribution to COVID-19 research
Wednesday, April 1, 2020,
In the COVID-19 emergency, ECRIN has established, with its national partners, a COVID-19 taskforce with its national partners, working on a fast-track procedure for access to, and provision of services:
- Review and digest the scientific literature on COVID clinical trials
- Develop a metadata repository for COVID trials making all the non-sensitive COVID-19 trial data accessible
- Develop a database on the regulatory, ethical and data protection fast track approvals across all European countries
- Ensure preparedness of its national clinical trial unit (CTU) partners for COVID trials
- Combine and coordinate national initiatives to promote multinational rather than national trials, including through connection with national funders, sponsors investigators and CTUs
- Develop partnership with national and pan-European investigation networks on infectious diseases and intensive care
- Outreach to investigators, sponsors, patients, policymakers, funders, and citizens
- International cooperation and outreach, including with WHO and through CRIGH and other initiatives.
For any further information please contact ECRIN’s Head of Clinical Operations Unit, Sabine Kläger via mail at sabine.klager@ecrin.org
#StopTheSpread
Monday, March 30, 2020,

Consistent with Covid-19 outbreak and subsequent measures adopted by the Italian Council of Ministers in conjunction with the Ministry of Health, no-profit Sponsors, CTUs/CROs and many pharmaceutical companies have applied or extended smart-working in order to continue their activities related to clinical trials and to assure at the same time the highest possible protection of the personnel involved. To this regard, please see the AIFA announcement.
A new CTU joined ItaCRIN Network!
Friday, February 21, 2020,
It is our great pleasure to announce that CD PHARMA became part of ItaCRIN.
Our Network is growing fast,
Welcome CD PHARMA!
EJP RD – RDR Challenges NETWORKING EVENT ANNOUNCEMENT
Friday, January 10, 2020,

Summary information on the RDR Challenges Call
The RDR Challenges call will be launched in March 2020.
It foresees selection, evaluation and funding of projects in a 2-stage process. Challenges must be solvable in a short time period of 30 months with first milestones/deliverables at M18. A first Scientific Evaluation Committee (SEC) meeting will evaluate applications responding to the proposed challenges for the first 18-month phase grant. The aim of this selection process is to fund one project for each proposed challenge. Following the completion of the first phase (after 18 months), the applicants submit a report demonstrating the work undertaken during the study and, if appropriate, an application for phase 2 funding. The SEC will examine the results from the 1st phase and evaluate what has been delivered in the specific timeline and how the applicants met the milestones/deliverables. After positive evaluation, the SEC will validate projects for the second phase grant.
The total budget of 1.5 Mio€ funding from the European Commission allows for 4 projects to be funded (375 000 € per project).
The involved industry partners who identified a challenge will join the consortium of applicants once selection is made by the SEC and will co-fund (in cash and in kind) the granted project.
THE NETWORKING EVENT FOR THE RDR CHALLENGES CALL WILL BE HELD ON 3rd MARCH 2020 IN PARIS
For more informations, please click here: http://www.ejprarediseases.org/index.php/funding-schemes/rare-diseases-challenges/
Networking Support Scheme is open
Tuesday, March 3, 2020,

The EJP RD (European Joint Program Rare Diseases) project has opened (on a continuous basis-no deadline) a “call” to provide financial support for organizing workshops to share knowledge on rare diseases.
Eligible applicants to apply for the NSS are health care professionals, researchers and patient advocacy organizations from the following countries involved in the EJP RD (in alphabetical order): Armenia*, Austria, Belgium, Bulgaria*, Croatia*, Czech Republic*, Denmark, Estonia*, Finland, France, Germany, Georgia*, Greece, Hungary*, Ireland, Israel, Italy, Latvia*, Lithuania*, Luxembourg, Malta*, Norway, Poland*, Portugal, Romania*, Serbia*, Slovakia*, Slovenia*, Spain, Sweden, Switzerland, The Netherlands, Turkey*, United Kingdom.
The application template with the link to the electronic proposal submission system
ProjectNet light can be found at the EJP RD website: http://www.ejprarediseases.org/index.php/networking-support/
ANNOUNCEMENT JOINT TRANSNATIONAL CALL 2020
Monday, December 16, 2019,
“Multidisciplinary Research Projects on Personalised Medicine –
Pre-/Clinical research, Big Data and ICT, Implementation and User’s Perspective”
ERA PerMed is an ERA-NET Cofund, supported by 32 partners of 23 countries and cofunded by the European Commission (EC). The funding organisations participating in this call particularly wish to promote innovative interdisciplinary collaboration and to encourage translational research proposals. The available budget for this call is 27 Mio € (approx.).
Opening of online submission tool: 16 December 2019
Submission deadline for pre-proposals: 5 March 2020 (17:00 CET)
Submission deadline for full-proposals: 15 June 2020 (17:00 CEST)
For more informations, click here: ERA PerMed website
November 2019 Newsletter is now online!
Friday, November 29, 2019,
ECRIN's newsletter provides relevant updates on ECRIN's work.
Click here for the new issue: November 2019
To sign up, please click here.
Istituto Superiore di Sanità
Viale Regina Elena, 299 - 00161 - Roma
